in basket
Pharma Solutions
Industry statistics indicate that:
- 25% of vaccines reach their destination in a degraded state because of incorrect shipping.
- Almost 30% of scrapped sales at pharmaceutical companies can be attributed to logistics issues.
- Almost 20% of temperature-sensitive health care products are damaged during transport due to a broken cold chain.
- Approximately 0.5% of transported goods are damaged during transport through non-compliance to temperature guidelines.
Maintaining the quality and integrity of medical and biological products during transport is critical for pharmaceutical manufacturers and logistics providers for public health and patient safety.
As most medical and biological products require a temperature-controlled environment at all stages of manufacturing and distribution, the control of storage and transportation temperatures is vital in maintaining the quality and effectiveness of medicines. We understand that if the quality of pharmaceutical product shipments is compromised, the risk is more than loss of cargo – it can compromise the health and well-being of patients.
What is Good Distribution Practice (GDP) for pharmaceutical products?
The objective of Good Distribution Practice (GDP) guidelines is to ensure that a high level of product quality, determined by good manufacturing practices, is maintained throughout the distribution chain. This extends beyond the transport vehicles used to take pharmaceuticals (such as APIs) and medical components from the manufacturing facility to distributors and wholesalers. It must also ensure compliant delivery to hospitals and pharmacies.
Distributors must comply with the European Union guidelines on good distribution practice of medicinal products for human use.
Ballinlough Refrigeration Ltd are 'GDP Champion Certified' to help with any of your pharmaceutical needs. It is necessary to ensure there is a high level of product quality, efficiency, safety, and traceability which is maintained throughout the distribution chain. Whatever your needs, we have a solution for you.
Temperature Mapping
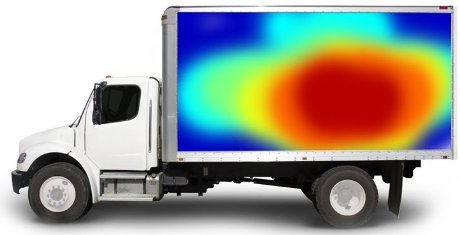
Temperature trailer mapping is a quality performance test for temperature-controlled trailers. However, experience shows that the programmed temperature is not always within the required limits leading to product quality damages and needs to be avoided.
Ballinlough Refrigeration Ltd can perform temperature mapping tests that pinpoint and visualises locations in the trailer where possible “hot-spots” appear. Trailer temperature mapping is therefore a performance test for your product quality. Every test is performed after agreeing on precise customer requirements and expectations, thus every temperature mapping is different. At the end of the process, a detailed report with a description of the temperature distribution, graphical presentation, and statistics is generated.
This service is intended for producers of medicines and components, research companies, pharmaceutics wholesalers, and chemist’s shops. Temperature mapping ensures having full knowledge of environmental conditions and can be the basis for qualification of means of transport by good distribution practice.
GDP Validation
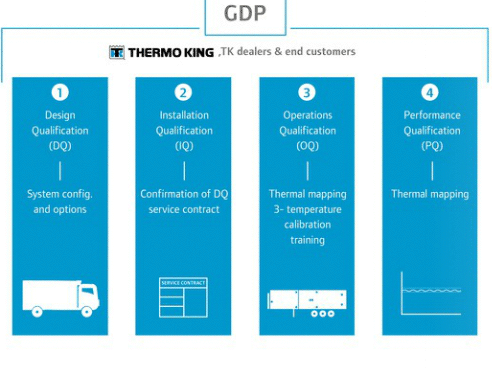
Thermo King developed a protocol covering the four stages outlined in the European Commission’s GDP guidelines of Medical products, including:
- Design Qualification
- Installation Qualification
- Operations Qualification
- Performance Qualification
PharmaSolutions offers a range of equipment for temperature-controlled transportation for trailers, trucks, VP units and ColdCube containers. All equipment has been tested following the Thermo King GDP validation protocol in line with GDP guidelines.